Determination of Specific Rotation of Sugar Solution Using a Half-Shade Polarimeter
Objective
To determine the specific rotation of a given sugar solution by measuring the angle of rotation of plane-polarized light using a half-shade polarimeter.
Apparatus Required
- Half-shade polarimeter
- Monochromatic light source (Sodium lamp, λ = 589.3 nm)
- Sugar solution of known concentration
- Measuring cylinder
- Glass tubes (Polarimeter tubes)
- Thermometer
- Distilled water (for calibration)
Diagram
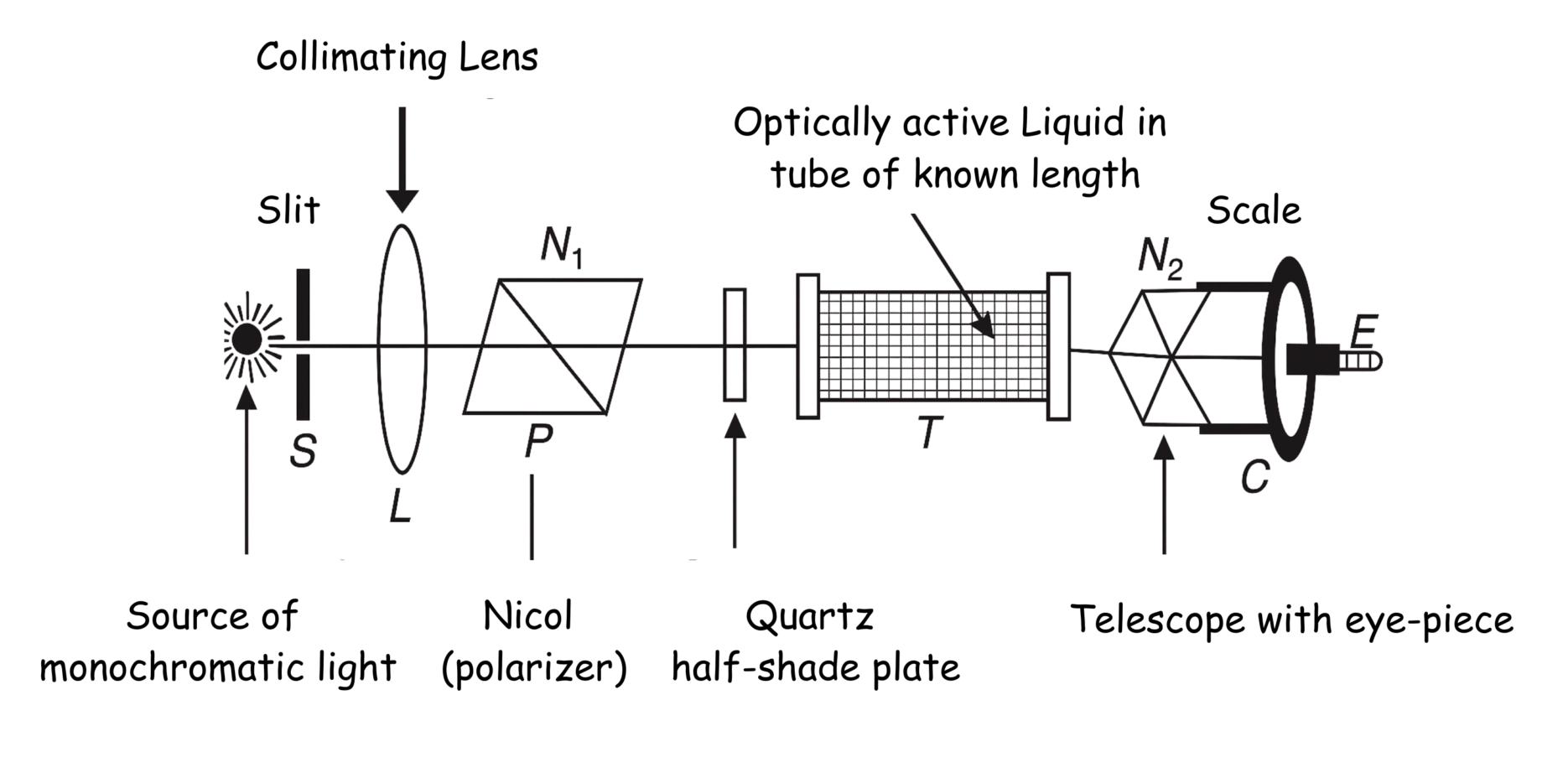
Theory
Optical Activity
Certain substances (like sugar) rotate the plane of polarization of plane-polarized light. This property is called optical activity.
Specific Rotation (α)
The specific rotation of a substance is defined as the angle of rotation (in degrees) produced by a solution of length 1 dm and concentration 1 g/cm³ at a given wavelength (usually sodium D-line, 589.3 nm) and temperature.
Where:
α = Specific rotation (degrees)
θ = Observed rotation (degrees)
l = Length of the tube (dm)
c = Concentration of the solution (g/cm³)
Half-Shade Polarimeter Principle
A polarimeter consists of a polarizer and an analyzer. The half-shade device splits the field of view into two halves, allowing for more precise determination of the angle at which the intensities of the two halves match.
Procedure
A. Calibration of Polarimeter (Zero Adjustment)
- Fill the polarimeter tube with distilled water and place it in the polarimeter.
- Adjust the analyzer until the two halves of the field appear equally bright (null position).
- Note the reading on the vernier scale as the zero position (θ₀).
B. Measurement of Angle of Rotation (θ)
- Prepare a known concentration (e.g., 10%) of sugar solution.
- Fill the polarimeter tube with the sugar solution, ensuring no air bubbles are trapped.
- Place the tube in the polarimeter and rotate the analyzer until the two halves of the field match in intensity.
- Note the new reading (θ₁).
- The observed rotation θ = θ₁ - θ₀.
- Repeat the experiment for different concentrations and take average readings.
Observations & Calculations
| S.No. | Concentration (c) (g/cm³) | Length (l) (dm) | Observed Rotation (θ) (degrees) | Specific Rotation (α) |
|---|---|---|---|---|
| 1 | 0.1 | 2.0 | [Insert value] | α = θ/(l×c) |
| 2 | 0.2 | 2.0 | [Insert value] | α = θ/(l×c) |
| 3 | 0.3 | 2.0 | [Insert value] | α = θ/(l×c) |
Average Specific Rotation (α_avg)
Result
Precautions
- Ensure the polarimeter tube is clean and free from air bubbles.
- Use monochromatic light (sodium lamp) for accurate results.
- Record temperature, as specific rotation is temperature-dependent.
- Take multiple readings for better accuracy.
- Handle the polarimeter gently to avoid misalignment.
Viva Questions with Detailed Answers
- Better End-Point Detection: In a simple polarimeter, determining the exact point of complete darkness is difficult due to gradual intensity changes. The half-shade device creates a sharp boundary between two half-fields of different intensities, making the null point easier to identify.
- Reduced Eye Strain: The human eye can more accurately detect when two adjacent fields have equal brightness rather than detecting absolute darkness, reducing fatigue and errors.
- Higher Precision: The sharp contrast between the two halves allows for more precise angle measurements, typically achieving accuracy of ±0.1° compared to ±0.5° in simple polarimeters.
- Elimination of Systematic Errors: The half-shade method reduces errors due to imperfect polarization and stray light effects.
θ = α × l × c
Where θ is the observed rotation, α is the specific rotation (a constant for a given substance at fixed temperature and wavelength), l is the path length, and c is the concentration.
Key Points:
- Doubling the concentration doubles the angle of rotation
- This linear relationship holds true for dilute solutions
- At very high concentrations, deviations may occur due to intermolecular interactions
- This proportionality allows us to determine unknown concentrations by measuring rotation angles
- Standard Reference: It serves as the international standard wavelength for reporting specific rotation values, ensuring consistency across different laboratories and measurements worldwide.
- Monochromatic Light: The D-line provides essentially monochromatic light, which is crucial because specific rotation varies with wavelength (optical rotatory dispersion). Using a single wavelength eliminates errors due to dispersion effects.
- High Intensity: Sodium lamps produce intense D-line emission, providing bright, easily observable light for accurate measurements.
- Availability: Sodium lamps are readily available, inexpensive, and commonly used in laboratories.
- Visibility: The yellow light (589.3 nm) is in the visible spectrum where the human eye has good sensitivity, making visual observations comfortable and accurate.
- Historical Standard: The D-line has been used historically, allowing comparison with literature values accumulated over many years.
Effects of Temperature:
- Molecular Motion: Increased temperature causes greater molecular motion, which can affect the interaction between light and optically active molecules, generally decreasing the specific rotation.
- Density Changes: Temperature affects solution density, which indirectly influences the effective concentration of the optically active substance.
- Conformational Changes: For complex molecules, temperature can cause conformational changes that alter optical activity.
- Solvent Effects: Temperature changes affect solvent properties, which can influence the solvation of optically active molecules.
Typical Temperature Coefficient: For most sugars, specific rotation decreases by approximately 0.01° to 0.03° per degree Celsius increase in temperature.
Standard Conditions: Specific rotation is typically reported at 20°C or 25°C, and temperature must be recorded and controlled for accurate and reproducible measurements. The notation [α]²⁰_D indicates specific rotation measured at 20°C using sodium D-line.
