Surface Tension Determination by Jaeger's Method
1. Aim
2. Apparatus Used
- Jaeger's apparatus (consisting of a pressure manometer and a capillary tube)
- Traveling microscope
- Mercury manometer or pressure sensor
- Thermometer
- Beaker
- Test liquid (water, alcohol, etc.)
- Screw gauge
- Magnifying glass
- Stopwatch
- Rubber tubing and connector
- Retort stand with clamps
- Spirit level
3. Diagram
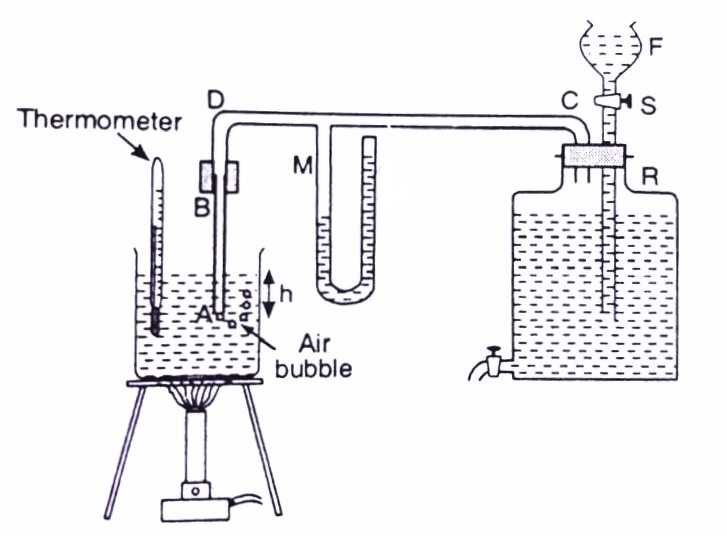
Fig: Schematic diagram of Jaeger's apparatus for measuring surface tension
4. Theory
The Jaeger's method for measuring surface tension is based on the principle that when air is forced through a capillary tube immersed in a liquid, the maximum pressure required to form a bubble is related to the surface tension of the liquid.
When the tip of a capillary tube is immersed in a liquid, the pressure required to form a bubble at the end of the tube depends on:
- The surface tension of the liquid
- The radius of the capillary tube
- The depth of immersion of the capillary tip
According to Jaeger's method, the maximum pressure (P) required to form a bubble at the end of a capillary tube of radius 'r' immersed to a depth 'h' in a liquid of density 'ρ' is given by:
Where:
- \(P_0\) is the atmospheric pressure
- \(\rho\) is the density of the liquid
- \(g\) is the acceleration due to gravity
- \(h\) is the depth of immersion
- \(T\) is the surface tension of the liquid
- \(r\) is the radius of the capillary tube
For two different depths of immersion \(h_1\) and \(h_2\), the difference in maximum pressures will be:
By measuring the maximum pressure at different depths, we can determine the surface tension of the liquid.
5. Formula
The surface tension (T) of the liquid is given by:
Where:
- \(P\) is the maximum pressure required to form a bubble
- \(\rho\) is the density of the liquid
- \(g\) is the acceleration due to gravity
- \(h\) is the depth of immersion
- \(r\) is the radius of the capillary tube
Alternatively, using Jaeger's simplified method with two different depths (\(h_1\) and \(h_2\)):
Where \(P_1\) and \(P_2\) are the maximum pressures at depths \(h_1\) and \(h_2\) respectively.
6. Procedure
Setup Preparation:
- Clean the apparatus thoroughly to remove any impurities.
- Fill the beaker with the test liquid.
- Mount the capillary tube vertically using a retort stand and clamp.
- Connect the capillary tube to the manometer using rubber tubing.
Measurement of Capillary Radius:
- Use a screw gauge to measure the diameter of the capillary tube at different positions.
- Take at least 5 readings and calculate the average radius.
Measurement of Surface Tension:
- Immerse the capillary tube in the liquid to a small depth (\(h_1\)).
- Measure this depth accurately using a traveling microscope.
- Gradually increase the pressure in the system until bubbles just begin to form at the tip of the capillary.
- Record the maximum pressure (\(P_1\)) indicated by the manometer at which bubbles are formed.
- Repeat the measurement 5 times and take the average value of \(P_1\).
Repeat for Different Depths:
- Change the depth of immersion to a new value (\(h_2\)).
- Measure this new depth accurately.
- Determine the maximum pressure (\(P_2\)) required for bubble formation at this depth.
- Repeat 5 times and calculate the average value of \(P_2\).
Temperature Measurement:
- Record the temperature of the liquid using the thermometer.
Calculations:
- Substitute the measured values in the formula to calculate the surface tension of the liquid.
7. Observation Table
Table 1: Measurement of Capillary Radius
| Reading No. | Screw Gauge Reading (mm) | Radius (r) = Diameter/2 (mm) |
|---|---|---|
| 1 | ||
| 2 | ||
| 3 | ||
| 4 | ||
| 5 | ||
| Average |
Table 2: Measurement of Pressure at Different Depths
| Reading No. | Depth h₁ (cm) | Maximum Pressure P₁ (N/m²) | Depth h₂ (cm) | Maximum Pressure P₂ (N/m²) |
|---|---|---|---|---|
| 1 | ||||
| 2 | ||||
| 3 | ||||
| 4 | ||||
| 5 | ||||
| Average |
- Temperature of liquid: ______ °C
- Density of liquid at experimental temperature: ______ kg/m³
- Acceleration due to gravity (g): 9.8 m/s²
8. Calculations
-
Calculate the average radius of the capillary tube:
\[ r = \frac{r_1 + r_2 + r_3 + r_4 + r_5}{5} \] -
Calculate the average maximum pressures at depths \(h_1\) and \(h_2\):
\[ P_1(avg) = \frac{P_{11} + P_{12} + P_{13} + P_{14} + P_{15}}{5} \] \[ P_2(avg) = \frac{P_{21} + P_{22} + P_{23} + P_{24} + P_{25}}{5} \] -
Calculate the surface tension using the formula:
\[ T = \frac{[P_2(avg) - P_1(avg) - \rho g(h_2 - h_1)]r}{2} \]
Sample Calculation:
Given:
- r = 0.5 mm = 0.0005 m
- h₁ = 2 cm = 0.02 m
- h₂ = 4 cm = 0.04 m
- P₁(avg) = 2000 N/m²
- P₂(avg) = 2400 N/m²
- ρ = 1000 kg/m³ (for water)
- g = 9.8 m/s²
Calculation:
9. Result
(Compare this with the standard value of the liquid at the same temperature, if available, and calculate the percentage error.)
